Abstract
Background: Vaso-occlusive crises (VOCs) are the hallmark of SCD. The cell adhesion molecule P-selectin plays a key role in the multicellular interactions that can lead to VOCs. In the SUSTAIN trial in adults, crizanlizumab 5.0 mg/kg, a humanized monoclonal antibody that blocks P-selectin, significantly reduced the median annualized rate of VOCs vs placebo and had a favorable safety profile (Ataga et al. N Engl J Med 2017).
Aim: To describe initial safety and efficacy results for patients (pts) with SCD aged 12-<18 yr treated with crizanlizumab 5.0 mg/kg, with or without hydroxyurea (HU), in the SOLACE-kids trial (ClinicalTrials.gov NCT03474965).
Methods: SOLACE-kids is a Phase II study to confirm and establish appropriate dosing and evaluate safety of crizanlizumab in pediatric pts with SCD (any genotype) and ≥1 VOC leading to a healthcare (HC) visit within 12 mo prior to screening. Pts (N≥100) are stratified by age: Group 1 (G1; 12-<18 yr), Group 2 (6-<12 yr) and Group 3 (6 mo-<6 yr). Part A of the trial will confirm and establish crizanlizumab dosing based on first-dose and multiple-dose pharmacokinetic (PK) results (targeting similar exposure to adults) and safety in each group; Part B will expand recruitment for pts and evaluate long-term safety and efficacy of the PK-confirmed dose. Crizanlizumab is administered on Day 1, Day 15, then every 4 wk (up to 2 yr). Primary endpoints are PK and pharmacodynamic parameters (after starting dose and multiple doses) and frequency of adverse events (AEs). Secondary endpoints include the annualized rate of VOCs leading to HC visit, annualized rate of hospitalizations/emergency room (ER) visits and additional safety measures. This analysis focuses on safety and efficacy data of G1 pts receiving crizanlizumab 5 mg/kg.
Results: As of 28 August 2020, 50 pts were enrolled in G1 of SOLACE-kids. Mean (SD) age of pts was 15.0 (1.92) yr, 29 (58%) were female, 44 (88%) had the HbSS genotype, 32 (64%) were Black/African American and 42 (84%) were receiving HU. Median (range) duration of exposure to crizanlizumab was 36.6 (6-98) wk; 44 (88%) pts received treatment for ≥26 wk.
The most commonly reported AEs were headache (n=14 [28%]), vomiting (n=12 [24%]) and back pain (n=9 [18%]). Grade ≥3 AEs were reported in 13 (26%) pts; most common were anemia (n=3 [6%]) and back pain (n=2 [4%]). Serious AEs were reported in 11 (22%) pts; none were deemed related to treatment.
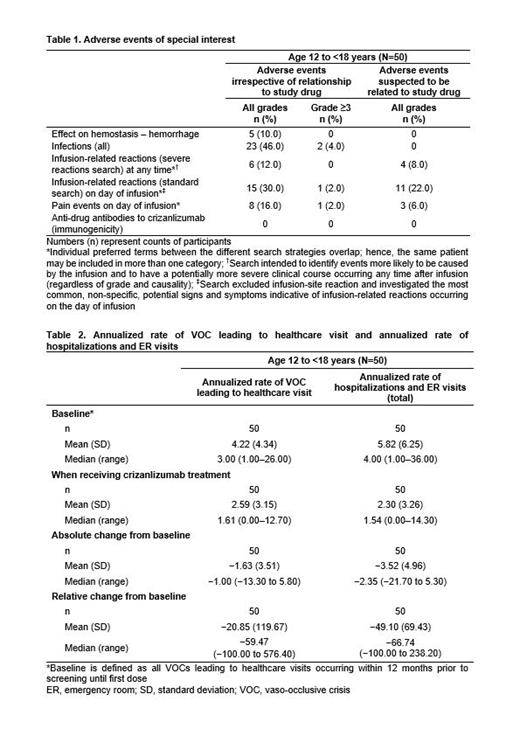
Incidence of AEs of special interest (AESI) is shown in Table 1. No AESI led to treatment discontinuation except 1 pt who died of meningitis (not related to treatment). No infusion-related reactions were serious; all had resolved at data cut-off (except for 1 case of Grade 1 dizziness). No case of anaphylactic reaction to crizanlizumab was reported. Pain events on the day of crizanlizumab infusion suspected to be related to treatment were reported in 3 (6%) pts. All pain events, regardless of relationship to treatment, were Grade 1/2, except for two Grade 3 events reported in the same pt (back pain and pain in extremity), which resolved on day of onset. All hemorrhage events were mild and not considered related to treatment.
Increase from baseline (BL) in total bilirubin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) was reported in 29 (58%), 17 (34%) and 20 (40%) pts, respectively. 2 (4%) pts had Grade 4 total bilirubin significantly above normal (1 pt was Grade 4 and 1 pt was Grade 3 at BL); 21 (42%) pts had Grade 3 total bilirubin significantly above normal (5 pts were Grade 3 at BL). Grade 3 increase in ALT and AST was reported in 2 (4%) pts each. All reported liver function parameters did not meet study criteria for severe drug-induced liver injury.
The median (range) number of VOCs leading to a HC visit was 3.0 (1.0-26.0) at BL and 1.6 (0.0-12.7) on treatment (median absolute reduction: 1.0 [range: -13.3 to 5.8]). 18 (36%) pts did not experience a VOC leading to a HC visit while on treatment. The median (range) annualized rate of hospitalizations/ER visits at BL was 4.0 (1.0-36.0) vs 1.54 (0.0-14.3) on treatment (median reduction: 2.35 [range: -21.7 to 5.3]) (Table 2).
Conclusion: This initial analysis of SOLACE-kids shows crizanlizumab 5.0 mg/kg is safe and well tolerated in pts aged 12-<18 yr, consistent with the established profile of crizanlizumab in adult pts. No new safety signals were identified. Compared with BL, crizanlizumab 5.0 mg/kg treatment led to a median reduction of 1 VOC leading to a HC visit/year in this pt population.
Heeney: FORMA: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Vertex / Crispr Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: DSMB; bluebird bio: Consultancy; Keros: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: DSMB; Cyclerion: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Rees: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Addmedica: Honoraria; TauRx: Membership on an entity's Board of Directors or advisory committees. De Montalembert: Vertex: Membership on an entity's Board of Directors or advisory committees; bluebird bio: Membership on an entity's Board of Directors or advisory committees; Addmedica: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Odame: Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Novartis: Other: Steering Committee; Global Blood Therapeutics: Other: DSMB. Brown: Imara: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Novo Nordisk: Consultancy; Forma Therapeutics: Research Funding; Pfizer: Research Funding; Global Blood Therapeutics: Consultancy, Research Funding. Wali: Novatis Oncology: Research Funding. Nguyen: Novartis: Current Employment. Lam: Novartis Pharmaceuticals Corporation: Current Employment, Current equity holder in publicly-traded company. Pfender: Novartis: Current Employment, Current equity holder in publicly-traded company. Kanter: Fulcrum Therapeutics, Inc.: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Forma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Agios: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beam: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Graphite Bio: Consultancy; GuidePoint Global: Honoraria; Fulcrum Tx: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal